Which subunit of the GABAB receptor obligate heterodimer controls cell suface stability and internalization of receptors?
Although many GPCRs are now known to oligomerize, GABAB receptors are unique because heterodimerization is indispensable for function of this GPCR. This is because R1 subunits contain the GABA binding site and R2 subunits contain the G-protein coupling site making these receptors obligate heterodimers.
Given that heterodimerization imparts unique biophysical properties to R1 and R2 monomers, we addressed which subunit of the monomer determine trafficking properties and cell surface stability of GABAB receptors .
We found that R2 subunits stabilize R1 subunits upon heterodimerization working via a dileucine motif in the C-terminus tail.
Heterodimerization enables R2 subunits to stabilize R1 subunits on the cell surface
By cloning a silent high-affinity epitope tag (an α-bungarotoxin binding site) on the N-terminus of R2 subunits and comparing the trafficking profile of R1 subunits engineered to express on the cell surface we found that heterodimerization stabilizes R1 subunits at the cell surface.
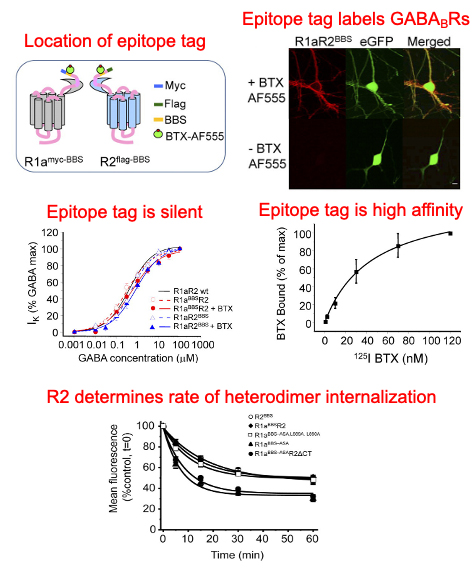
Dileucine motif on R1 determines the rate of internalization of the subunit
A dileucine motif in the R1 coiled-coil domain (R1a; Leu889, Leu890) acts as a dominant endocytic signal, and upon heterodimerization with R2, this motif is inactivated via an interaction of the coiled-coil domains, thereby increasing the stability of the heterodimer on the cell surface.

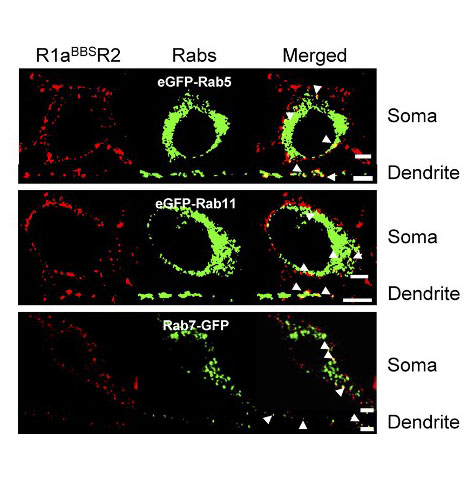
Trafficking itinerary of GABAB receptors after internalization
The fate of the internalized receptors was assessed by co-staining for intracellular structures in hippocampal neurons. Internalized GABAB receptors co-localized with Rab5, Rab11, and Rab7 containing intracellular compartments in the soma and dendrites of cultured hippocampal neurons.
Therefore, after being internalized, GABAB receptors are recruited to early endosomes, from where they are either recycled via recycling endosomes to be reinserted into the plasma membrane or are degraded in the lysosomes via the late endosomes.
New dual labelling strategy reveals that GABAB receptors are constitutively internalized as heterodimers
To develop a dual labelling strategy using α-bungarotoxin, we substituted two vicinal serines in the α-bungarotoxin (BTX) binding site (BBS) of R1BBS for cysteines (R1aBBS-CC).
By covalent labeling of the Cys residues using a sulfhydryl reagent (MTSES), the binding of BTX to R1 was prevented, whereas binding to the wild-type BBS on R2 could proceed unhindered. Subsequent removal of MTSES and exposure to a second flourophore-conjugated BTX allowed labelling of R1 subunits.
Using this strategy we found that R1 and R2 subunits are internalized as heterodimers.
To find out more about the dual labelling strategy visit this page.
