Why are certain GABAA receptor subunits expressed on the cell surface but others are not?
α subunits form a part of the GABA binding site and are indispensable GABAA receptor function. The six highly conserved α subunit isoforms (α1-6) are trapped in the endoplasmic reticulum and require oligomerisation with β or βγ/δ subunits to express on the cell surface.
Here we report that α subunits form homomeric ion channels on biological membranes and mutating just a single residue (either a key neurosteriod binding site or a volatile anaesthetic binding site) can express these subunits on the cell surface. Our results provide unique insights into the assembly and cell surface expression of GABAA receptor subunits.
The approach
While α subunits do not express on the cell surface on their, a handful of other subunits including ρ1 subunits (previously termed GABAC receptors) are able to access the plasma membrane.
By making chimeras of various combinations of α1 (the predominant cortical α subunit) with ρ1 , we indentified two discreet regions on α subunits that retain α subunits in the endoplasmic reticulum.
Unusually, switching the first and third transmembrane domains of α subunits with those ρ1 subunits allowed cell surface expression.
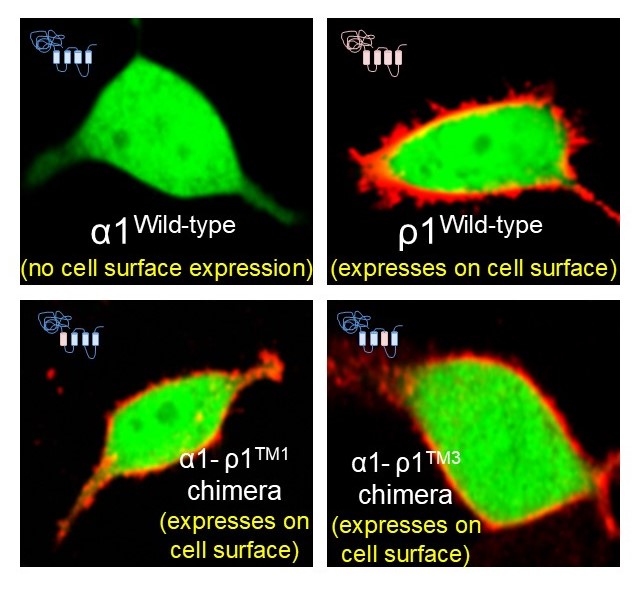
Substituting two single amino acids allows cell surface expression of α subunits
Using site directed mutagenesis, we identified two single amino acids that control plasma membrane expression of α subunits.
Q241 in the first transmembrane domain which forms a part of the neurosteroid binding site on GABAA receptors when mutated to an tryptophan (Q241W) allows cell surface expression.
A290 in the third transmembrane domain which forms a part of the volatile anaesthetic binding site on GABAA receptors when mutated to a tryptophan (A290W) also allows cell surface expression.

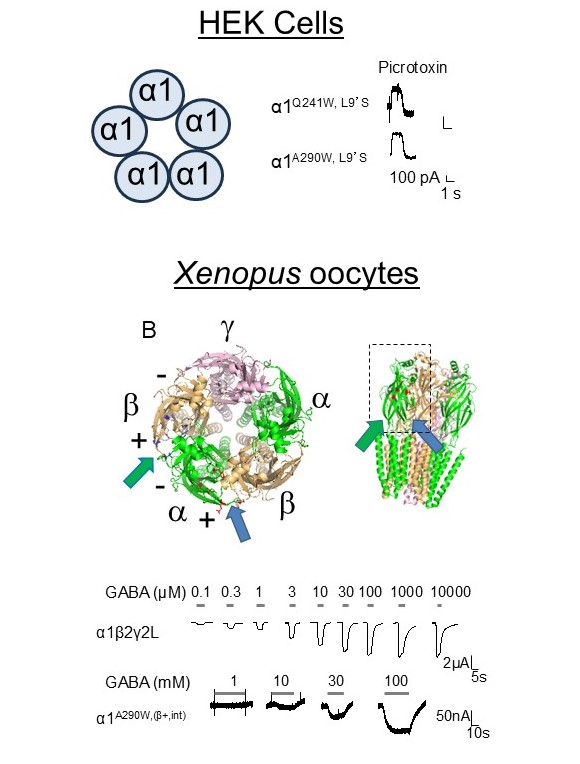
α subunit homomers form pentameric ion channels when expressed on the cell surface
By introducing a spontaneous gating mutation (L9’S) we found that α homomers can form spontaneously active pentameric ion channels on the plasma membrane.
In addition, by mutating one the interfaces of α subunits to resemble the GABA binding site found on β subunits, we imparted, albeit reduced, GABA sensitivity to α homomers expressed in Xenopus oocytes.
For more details please visit the publication:
*corresponding authors