Which part of the protein determeines stability of GABAB receptors on the cell surface?
GABAB receptors are obligate heterodimers composed of GABABR1 and R2 subunits. The two predominant R1 isoforms differ by the presence of two complement control protein modules or Sushi domains in the N terminus of R1a. This makes GABAB recepotors unusual as complement control protein domains are found only in a handful of GPCRs.
Sushi domains in R1a function as axonal transport signals and heteromers formed from R1aR2 and R1bR2 are thought to play distinct roles in neurotransmission, with R1aR2 contributing to presynaptic heteroreceptors to inhibit neurotransmitter release, although both R1aR2 and R1bR2 populate postsynaptic membranes to dampen excitability.
Here we found that the two Sushi domains impart greater plasma membrane stability to GABAB receptors at the soma, dendrites and dendritic spines.
The approach
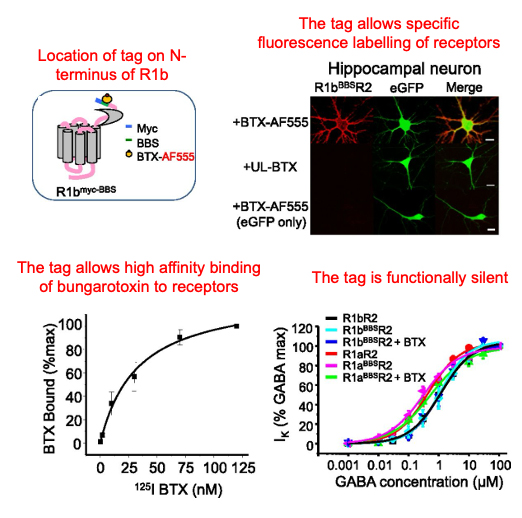
We cloned an α-bungarotoxin binding site on the R1b subunit.
This allowed high affinity fluorescence labelling of R1b-containing receptors.
The epitope tag was functionally silent
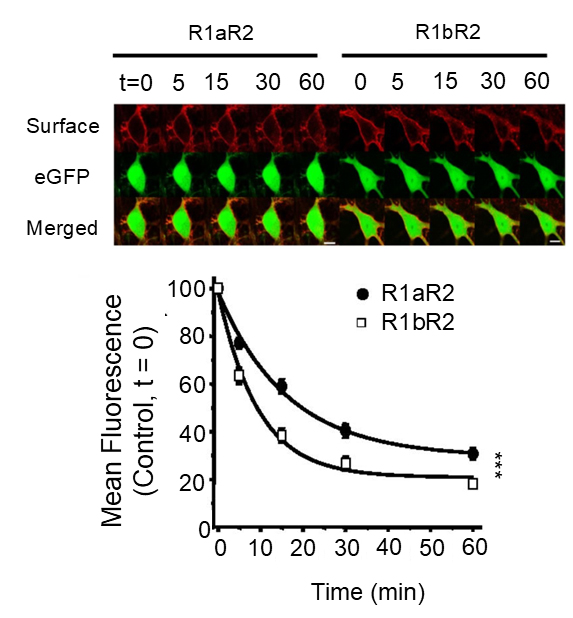
Sushi domains reduce internalization of GABAB receptors in the soma
Presence of the Sushi domains in R1aR2 enables these receptors to be more stabel on the cell surface than R1bR2 receptors
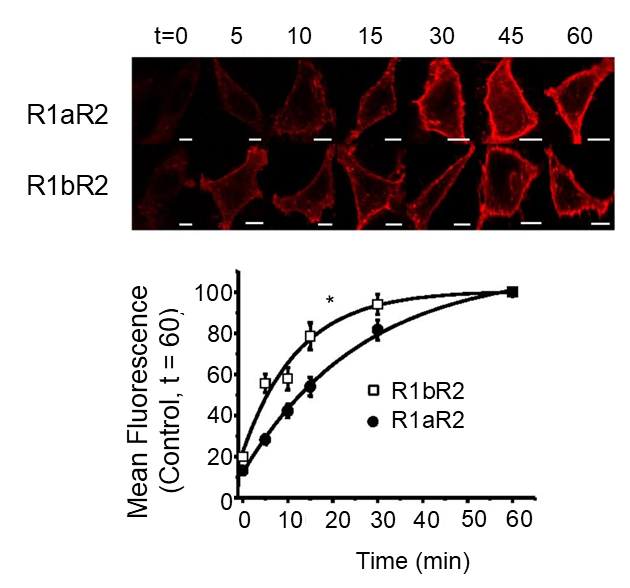
Sushi domains reduce membrane insertion rates of GABAB receptors
Presence of the Sushi domains in R1aR2 enables these receptors to be more stabel on the cell surface than R1bR2 receptors
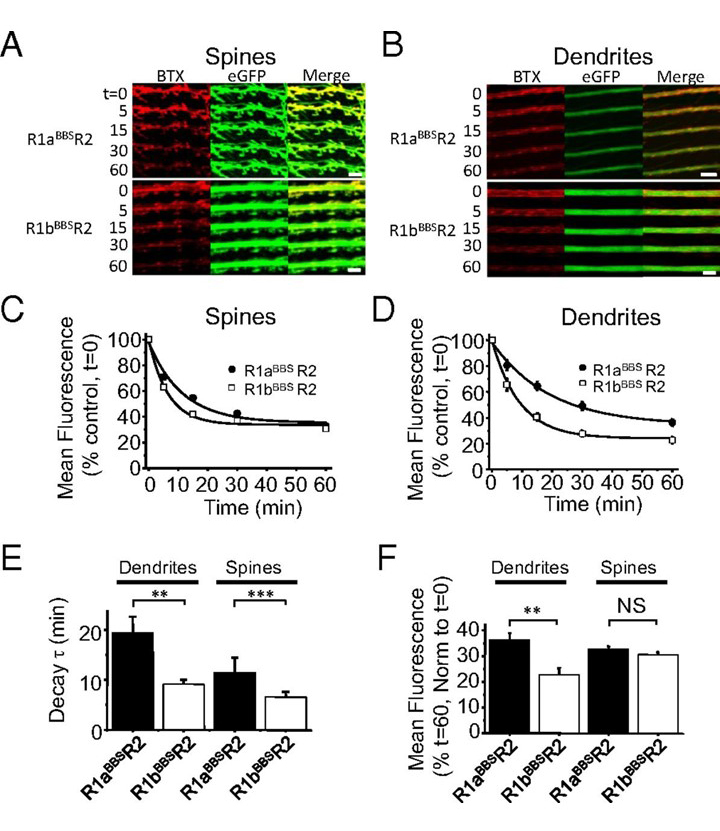
Sushi domains reduce internalization of GABAB receptors in the dendrites and spines
Presence of the Sushi domains in R1aR2 enables these receptors to be more stabel on the cell surface of dendrites and spines than R1bR2 receptors
Addition of the Sushi domains to mGluR2 reduces internalization of metabotropic glutamate receptors
We inserted an α-bungarotoxin binding site in metabotropic glutamate receptor 2 (mGluR2) which are in the same class C family of GPCRs as GABAB receptors. The epitope tag allowed labelling of mGluR2s.
Addition of the GABAB receptor Sushi domains to mGluR2 enabled these receptors to be more stable on the cell surface cofirming the stabilizing influence of Sushi domains on cell surface.
