Here, we describe a minimal epitope-tagging method, based on the incorporation of an α-bungarotoxin binding site (BBS) into the GABAB receptor, to study receptor internalization in live cells using a range of imaging approaches.
Advantages of the α-bungarotoxin binding site
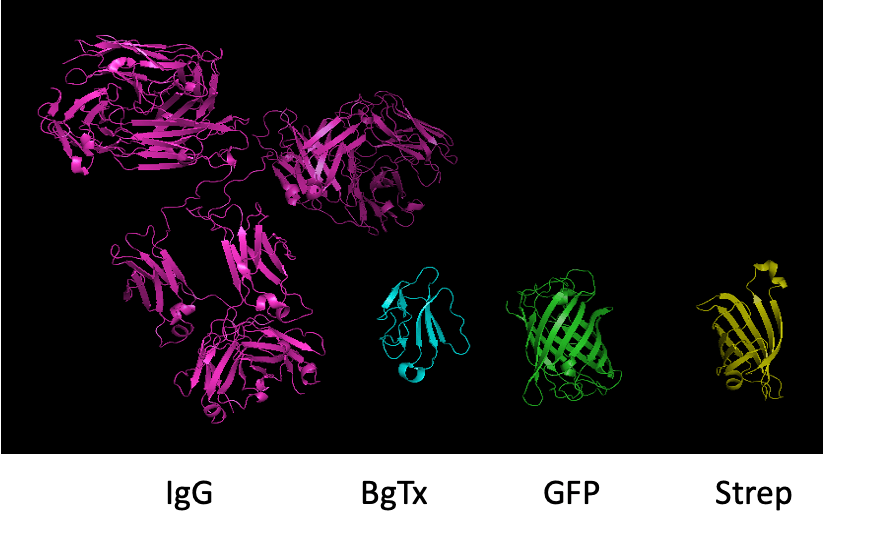
BTX-binding strategy has several advantages as a tagging method. Primarily, the BBS is relatively small (13 amino acids) compared to the size of green fluorescent protein (GFP; 238 amino acids), which is the most widely used fluorophore to label proteins.
The BBS is comparable in size to myc- (10 amino acids), flag- (8 amino acids), HA- (hemaglutinin, 9 amino acids), AP- (alkaline phosphatase, 15 amino acids) epitope tags, but these tags all require large, specific antibodies to be visualized, whereas the BBS needs only BTX and an organic fluorophore (e.g., rhodamine), which when complexed is still significantly smaller than antibody F(ab′)2 fragments and GFP.
Live cell imaging studies using antibodies have most frequently used primary antibodies allied to a complementary reaction with secondary antibodies or F(ab′)2 fragments coupled to organic fluorophores. These complexes are significantly larger in size compared to the BTX–fluorophore combination. Furthermore, BTX labeling is advantageous because, in comparison to fluorescent fusion proteins, it permits the discrimination of cell surface receptors from those located intracellularly.
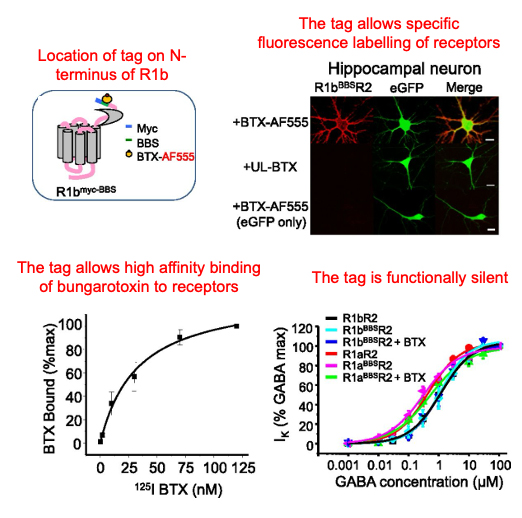
α-bungarotoxin binding sites can be high affinity and functionally neutral
We have engineered the α-bungarotoxin binding site in multiple GPCRs and these can be functionally neutral allowing high affinity labelling of receptors in various types of cells for live imaging studies.